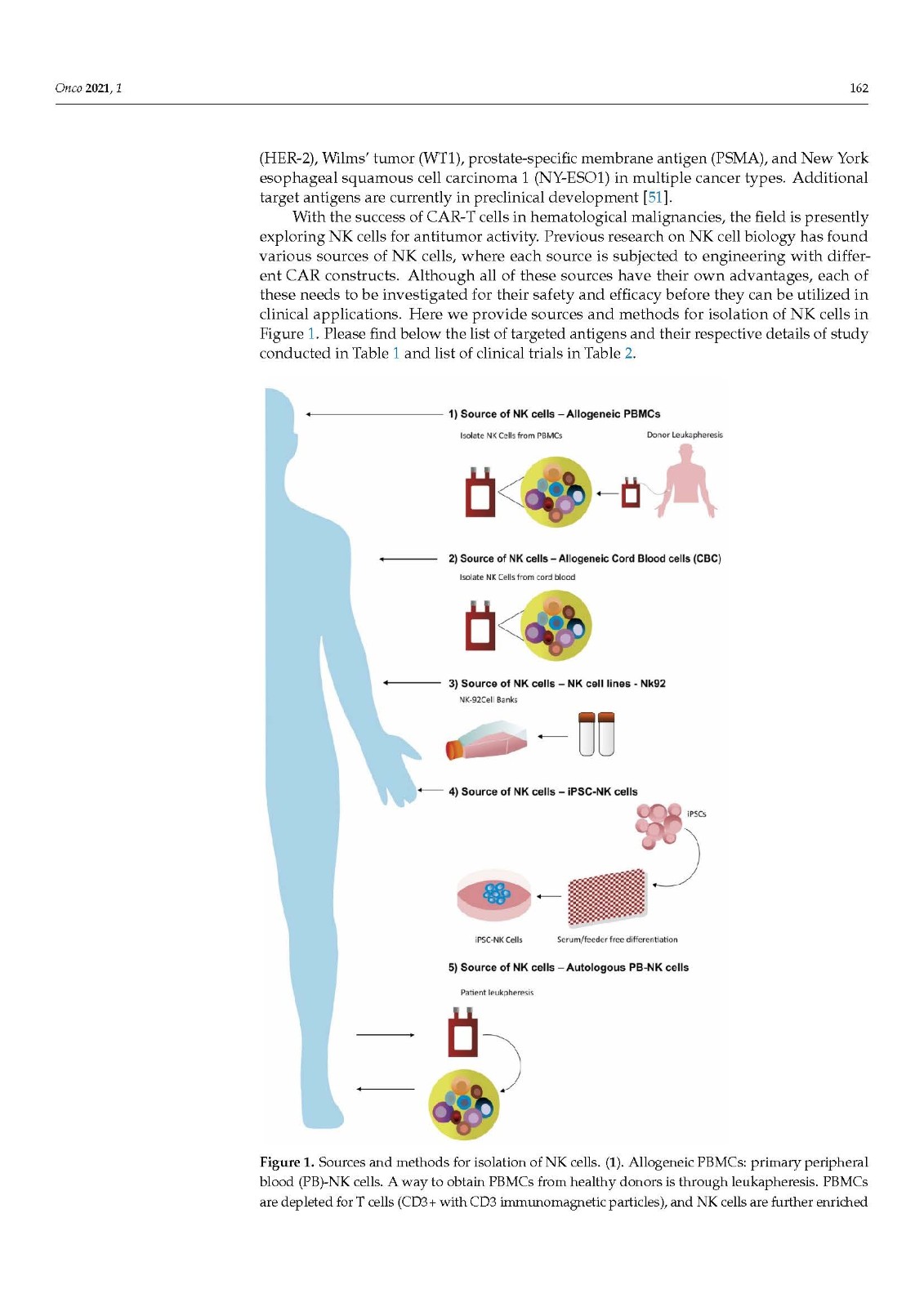
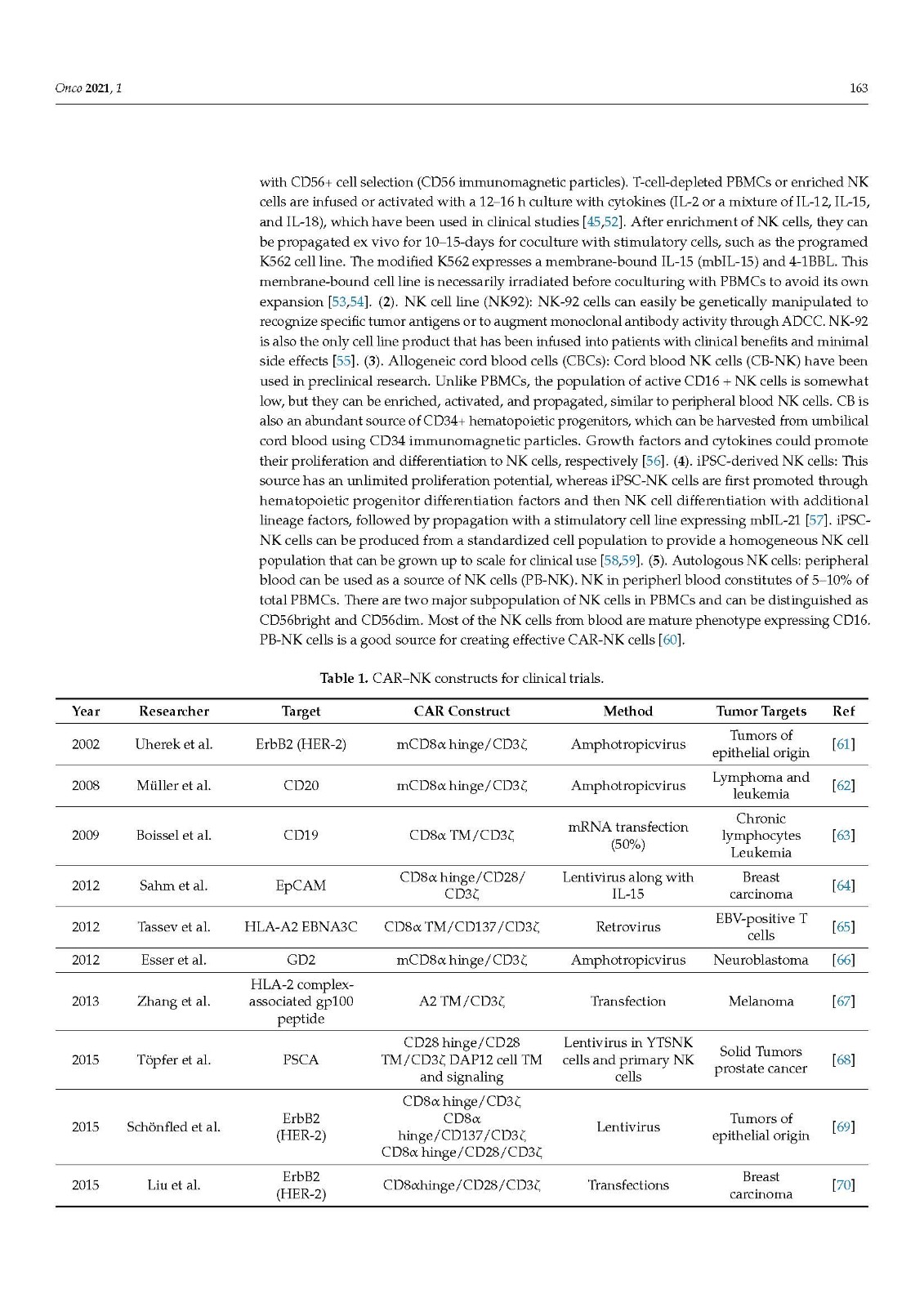
Natural killer cells are the first line of defense against tumor surveillance. Currently, efforts are being made to harness the potential of NK cells as tools for immunotherapy. Rapid progress is being made in engineering NK cells for tumor immunotherapy. Chimeric antigen receptor (CAR)-engineered NK (CAR-NK) cells are gaining ground on CAR-T cells because of better safety, cytotoxic function, and high feasibility for “off-the-shelf” manufacturing. In addition, bispecific NK cell engagers (BiKEs) and trispecific NK cell engagers (TriKEs) therapy have proven beneficial in solid tumors. In this review, we will focus on recent progress in the therapeutic competence of NK cell immunotherapy, including CAR-NK and BiKE across several in vivo and in vitro models.

T75细胞培养瓶
自然杀伤细胞是抵御肿瘤监视的第一道防线。目前,正在努力利用 NK 细胞作为免疫治疗工具的潜力。用于肿瘤免疫治疗的 NK 细胞工程正在取得快速进展。嵌合抗原受体 (CAR) 工程化 NK (CAR-NK) 细胞由于具有更好的安全性、细胞毒性功能以及“现成”制造的高可行性,在 CAR-T 细胞上取得了进展。此外,双特异性 NK 细胞接合剂 (BiKEs) 和三特异性 NK 细胞接合剂 (TriKEs) 疗法已被证明对实体瘤有益。在这篇综述中,我们将重点关注 NK 细胞免疫疗法的治疗能力的最新进展,包括 CAR-NK 和 BiKE 在多个体内和体外模型中的应用。
Increasing knowledge of cancer immunology has led to the design of therapies using immune cells directly or manipulating their activity, collectively termed immunotherapy. In the field of immuno-oncology, research on adaptive immune T cells has led to the development of CAR-T cells. Innate immune cells such as NK cells can also eliminate oncogenically transformed cells and regulate cells of the immune system. Considering NK cells as a live drug, numerous methods for the isolation and activation of NK cells have been shown to be clinically and therapeutically relevant. In such processes, various cytokines and antibodies present a source of stimulation of NK cells and enhance the efficacy of such treatments. The ex vivo expansion and activation of NK cells, along with genetic modification with CAR, enhance their antitumor activity. Recent preclinical studies have shown an antitumor effect through extracellular vesicles (EVs) derived from NK cells. Work with autologous NK cells has provided insights for clinical applications. In this review, we outline the recent advances of NK-cell-based immunotherapies, summarizing CAR-NK cells, BiKEs, and TriKEs as treatment options against cancer. This review also discusses the challenges of NK cell immunotherapy.
癌症免疫学知识的增加导致了直接使用免疫细胞或操纵其活性的疗法的设计,统称为免疫疗法。在免疫肿瘤学领域,对适应性免疫T细胞的研究带动了CAR-T细胞的发展。天然免疫细胞如 NK 细胞也可以消除致癌转化细胞并调节免疫系统细胞。将 NK 细胞视为一种活性药物,许多分离和激活 NK 细胞的方法已被证明具有临床和治疗相关性。在这些过程中,各种细胞因子和抗体是 NK 细胞的刺激源,并增强了此类治疗的功效。NK 细胞的体外扩增和激活,以及 CAR 的基因改造,增强了它们的抗肿瘤活性。最近的临床前研究表明,来自 NK 细胞的细胞外囊泡 (EV) 具有抗肿瘤作用。使用自体 NK 细胞为临床应用提供了见解。在这篇综述中,我们概述了基于 NK 细胞的免疫疗法的最新进展,总结了 CAR-NK 细胞、BiKE 和 TriKE 作为抗癌治疗选择。本综述还讨论了 NK 细胞免疫疗法的挑战。

40层细胞工厂
The future of clinical trials depends on many factors—most importantly, the trial design. NK cell immunotherapies, which harness the power of NK cells, have shown promising results and are complementary to T-cell therapies. Recent advances in gene manipulation have allowed for the creation of novel CAR-NK cell products with enhanced antitumor response [25]. The clinical trials of pleiotropic CAR-NK, which have gained a lot of attention, have begun to investigate the potential of this type of cancer immunotherapy. Similar to T cells, the engineering of NK cells continues to face difficulties such as safety, clinical efficacy, homing, dose replications, and in vivo persistence after treatment. Various modes of drug delivery, including lipid nanoparticles (LPNs) and liposomes, are currently being explored for use in adoptive transfer modalities. Briefly, LPNs are composed of ionizable lipid, a cholesterol moiety, and an additional helper phospholipid and lipid-anchorage polyethylene-glycol conjugate to minimize LPN aggregation [107]. Margert et al. reported LPNs designed for ex vivo mRNA delivery to human T cells. Several ionizable lipids were produced and then formulated into LPNs. They were further used to deliver mRNA to Jurkat cells, and some formulations were capable of enhancing mRNA delivery over lipofectamine. One of the LPN formulations was further selected to deliver CAR mRNA into primary human T cells. CAR-T cells engineered with the LPN method were then compared to electroporated (EP) CAR-T cells in a coculture assay with the Nalm-6 cell line, where CAR-T cells engineered via both these methods showed pronounced antitumor activity. Overall, this report signifies the importance of an alternate delivery method of mRNA to primary human T cells through LPN which can induce functional protein expression, as well as enhance mRNA-based CAR-T cells. Further investigation is required for the ability of LPNs to deliver cargo in TME and expression patterns in solid tumors [108].
Ultimately, the goal has been to provide an “off-the-shelf” product, which is considered nontoxic to normal healthy tissues after being adoptively infused into patients. The goal further covers the product that could be delivered to all HLA haplotypes without the occurrence of GvHD. Modification of iPSCs with CRISPR technology or inclusion of novel genes to improvise persistence of CAR-NK in TME are some of the key innovations moving forward. The reprogramming of CAR-NKs to overcome tumor suppression and escape is instrumental for the clinical outcome of NK therapies. Suitable gene modification along with biomanufacturing and banking processes will require further intricate optimization. Exploration for more optimal strategies and broad-range tumor targets for solid tumors is required. Manifestations of all these efforts are primarily aimed to avoid off-target cytotoxicity.

高效摇瓶5L
临床试验的未来取决于许多因素——最重要的是试验设计。利用 NK 细胞的力量的 NK 细胞免疫疗法已显示出有希望的结果,并且是 T 细胞疗法的补充。基因操作的最新进展允许创造具有增强抗肿瘤反应的新型 CAR-NK 细胞产品 [ 25]]。备受关注的多效性CAR-NK的临床试验,已经开始探索这种癌症免疫疗法的潜力。与 T 细胞类似,NK 细胞的工程化继续面临安全性、临床疗效、归巢、剂量复制和治疗后体内持久性等困难。目前正在探索各种药物递送模式,包括脂质纳米颗粒 (LPN) 和脂质体,以用于过继转移模式。简而言之,LPN 由可电离的脂质、胆固醇部分和额外的辅助磷脂和脂质锚定聚乙二醇共轭物组成,以最大限度地减少 LPN 聚集 [ 107]]。玛格特等人。报道了设计用于将 mRNA 离体传递到人类 T 细胞的 LPN。产生了几种可电离的脂质,然后将其配制成 LPN。它们被进一步用于将 mRNA 递送至 Jurkat 细胞,并且一些制剂能够增强 mRNA 递送而不是 lipofectamine。进一步选择了一种 LPN 制剂将 CAR mRNA 递送到原代人类 T 细胞中。然后在与 Nalm-6 细胞系的共培养试验中,将用 LPN 方法改造的 CAR-T 细胞与电穿孔 (EP) CAR-T 细胞进行比较,其中通过这两种方法改造的 CAR-T 细胞显示出明显的抗肿瘤活性。总的来说,这份报告表明了一种通过 LPN 将 mRNA 传递给原代人类 T 细胞的替代方法的重要性,该方法可以诱导功能性蛋白质表达,并增强基于 mRNA 的 CAR-T 细胞。108 ]。
最终,目标是提供一种“现成的”产品,这种产品在被过继注入患者体内后被认为对正常健康组织无毒。该目标进一步涵盖了可以在不发生 GvHD 的情况下交付给所有 HLA 单倍型的产品。使用 CRISPR 技术修改 iPSC 或包含新基因以提高 CAR-NK 在 TME 中的持久性是一些关键创新。CAR-NKs 的重编程以克服肿瘤抑制和逃逸对 NK 疗法的临床结果有帮助。合适的基因修饰以及生物制造和银行流程将需要进一步复杂的优化。需要探索针对实体瘤的更优化策略和更广泛的肿瘤靶点。
关键词:natural killer (NK) cells,cancer,immunotherapy,chimeric antigen receptor (CAR)-NK,iPSCs,bispecific T-cell engagers (BiKEs),trispecific NK cell engagers (TriKEs),自然杀伤(NK)细胞,癌症,免疫疗法,嵌合抗原受体 (CAR)-NK,iPSC,双特异性 T 细胞接合器 (BiKE),三特异性 NK 细胞接合器 (TriKE)















来源:MDPI https://www.mdpi.com/2673-7523/1/2/13/htm
上一篇: 血清瓶有哪些特点
下一篇: 细胞摇瓶培养液体菌种的五点优势