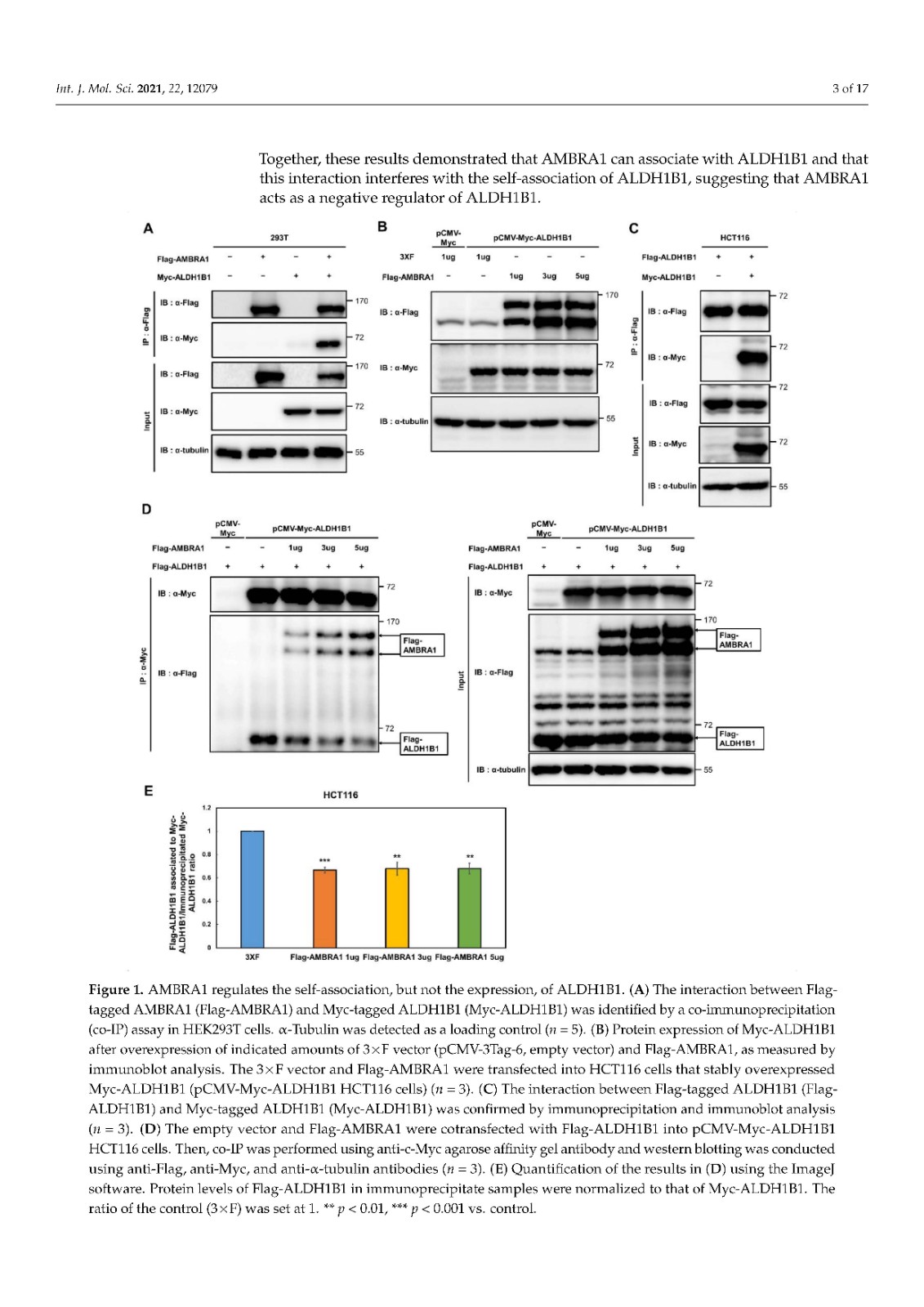
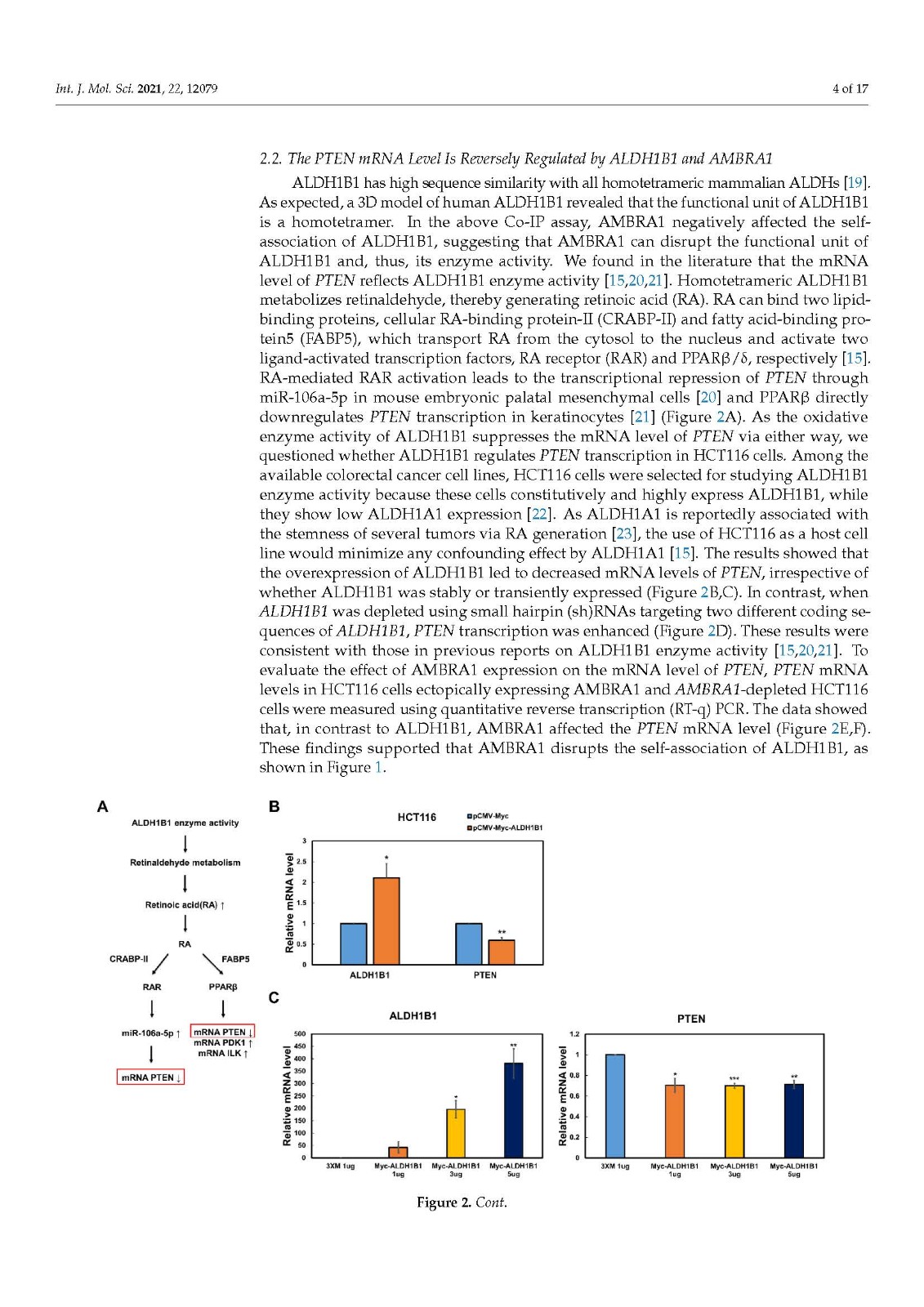
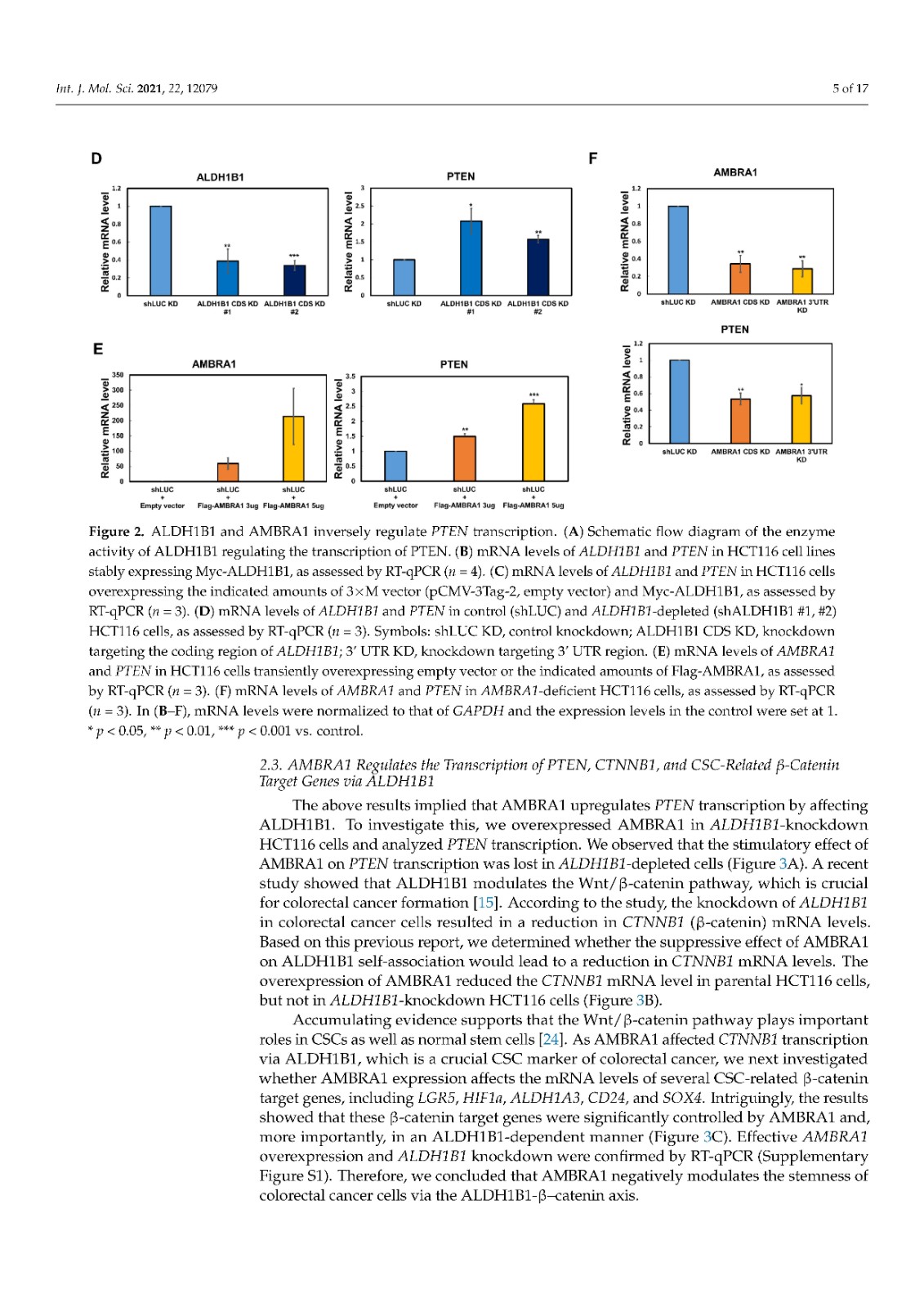
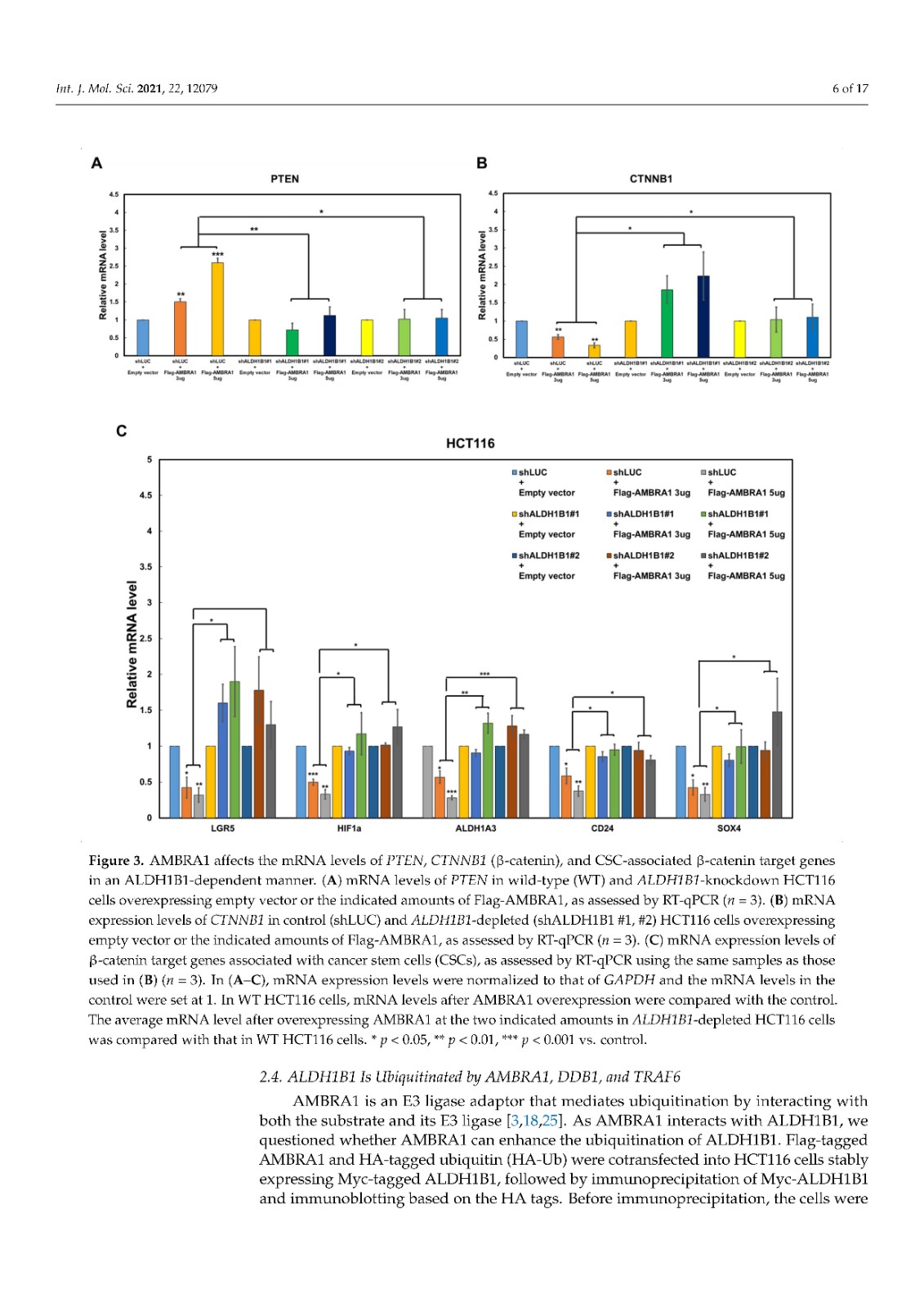
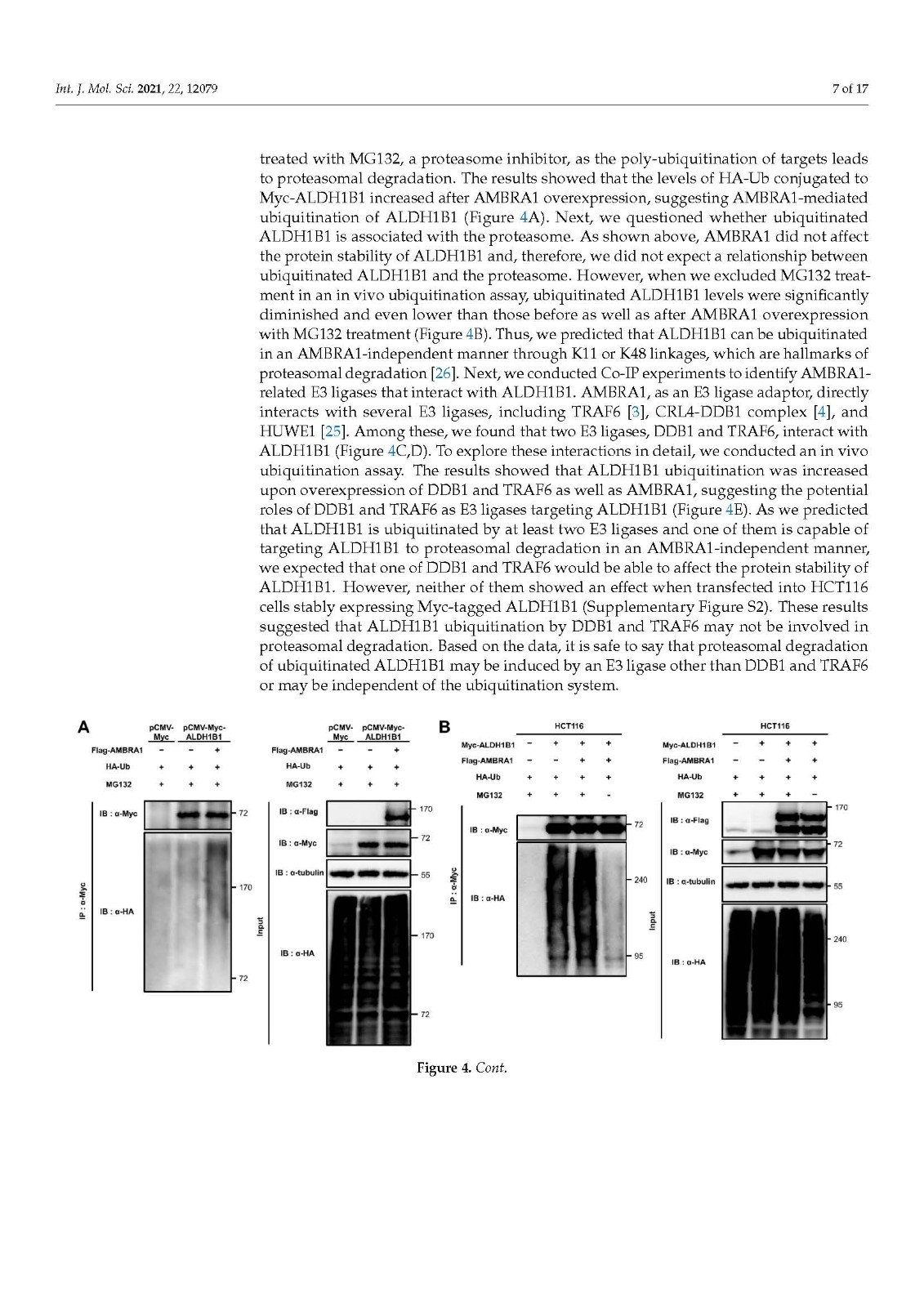
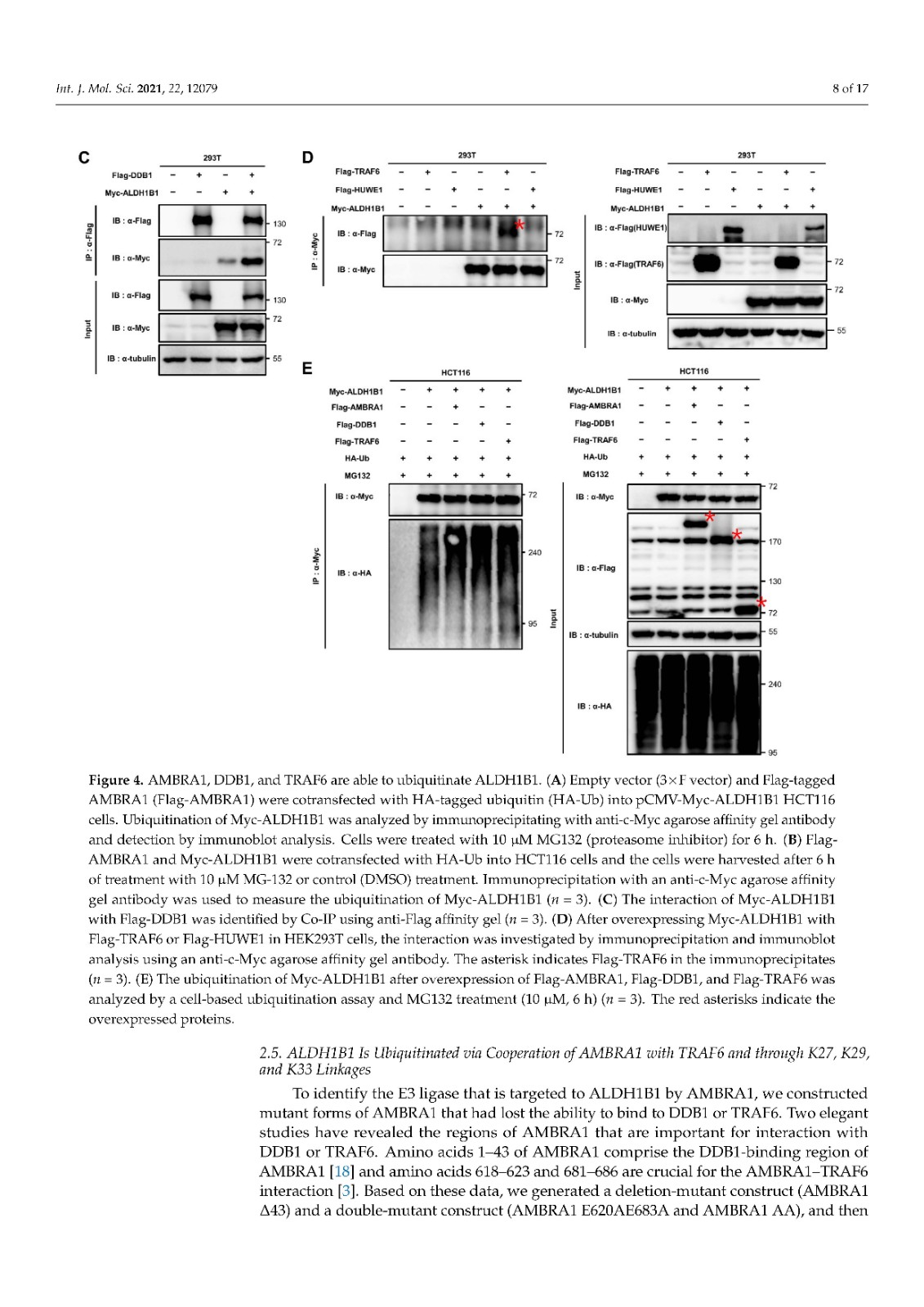
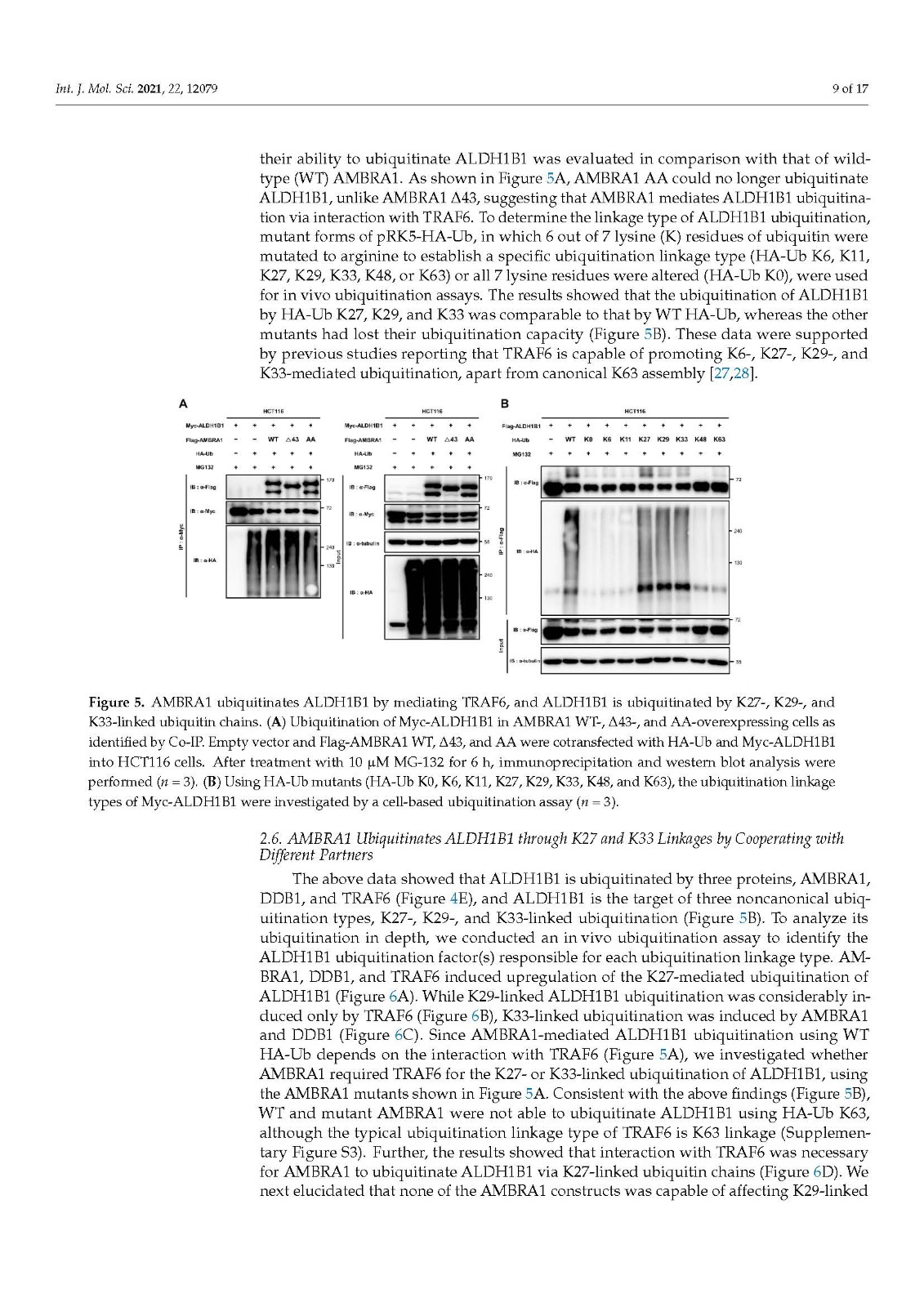
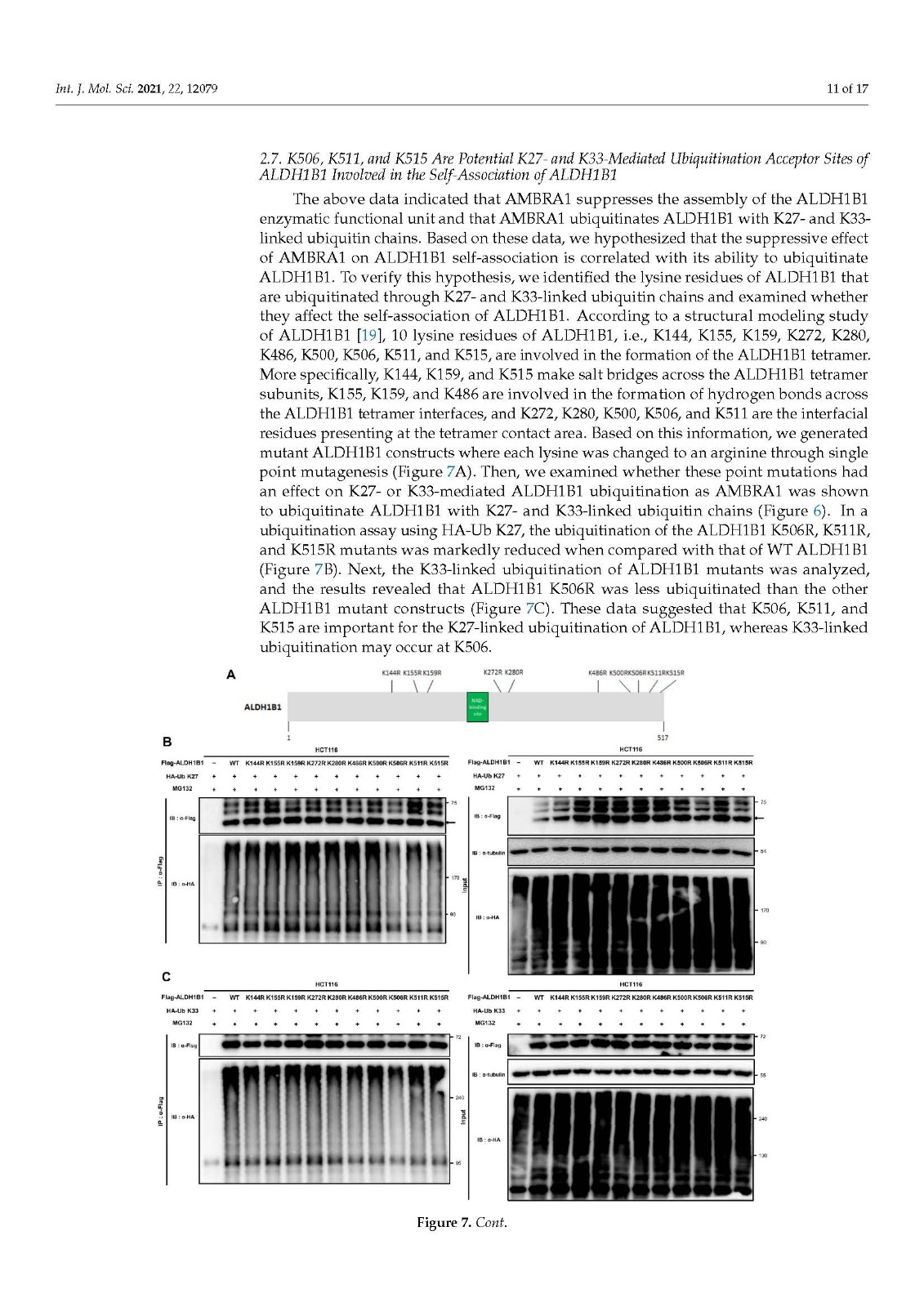
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global pandemic with a great impact on social and economic activities, as well as public health. In most patients, the symptoms of COVID-19 are a high-grade fever and a dry cough, and spontaneously resolve within ten days. However, in severe cases, COVID-19 leads to atypical bilateral interstitial pneumonia, acute respiratory distress syndrome, and systemic thromboembolism, resulting in multiple organ failure with high mortality and morbidity. SARS-CoV-2 has immune evasion mechanisms, including inhibition of interferon signaling and suppression of T cell and B cell responses. SARS-CoV-2 infection directly and indirectly causes dysregulated immune responses, platelet hyperactivation, and endothelial dysfunction, which interact with each other and are exacerbated by cardiovascular risk factors. In this review, we summarize current knowledge on the pathogenic basis of thromboinflammation and endothelial injury in COVID-19. We highlight the distinct contributions of dysregulated immune responses, platelet hyperactivation, and endothelial dysfunction to the pathogenesis of COVID-19. In addition, we discuss potential therapeutic strategies targeting these mechanisms.
由严重急性呼吸系统综合症冠状病毒 2 (SARS-CoV-2) 引起的 2019 年冠状病毒病 (COVID-19) 已成为全球流行病,对社会和经济活动以及公共卫生产生重大影响。在大多数患者中,COVID-19 的症状是高烧和干咳,并在 10 天内自然消退。然而,在严重的情况下,COVID-19会导致非典型双侧间质性肺炎、急性呼吸窘迫综合征和全身血栓栓塞,导致多器官功能衰竭,死亡率和发病率很高。SARS-CoV-2 具有免疫逃避机制,包括抑制干扰素信号传导和抑制 T 细胞和 B 细胞反应。SARS-CoV-2 感染直接和间接导致免疫反应失调、血小板过度活化和内皮功能障碍,它们相互作用并因心血管危险因素而加剧。在这篇综述中,我们总结了当前关于 COVID-19 血栓炎症和内皮损伤的致病基础的知识。我们强调了失调的免疫反应、血小板过度活化和内皮功能障碍对 COVID-19 发病机制的独特贡献。此外,我们讨论了针对这些机制的潜在治疗策略。
Based on the results of clinical trials, the current recommendation for the treatment of severely ill patients with COVID-19 receiving mechanical respiratory or circulatory supports is the use of dexamethasone. If these patients are within 24 h of admission to the ICU, dexamethasone plus tocilizumab, an IL-6 inhibitor, could be another option. For the treatment of hospitalized COVID-19 patients requiring oxygen delivery through a high-flow device or noninvasive ventilation, dexamethasone or dexamethasone plus remdesivir is recommended, while tocilizumab or baricitinib, a JAK inhibitor, can be added if rapidly worsening respiratory distress and systemic inflammation are observed in these patients. For hospitalized mild-to-moderate COVID-19 patients requiring supplemental oxygen, the use of dexamethasone and/or remdesivir is recommended. For COVID-19 inpatients without oxygen support or outpatients, there is insufficient evidence for therapeutic recommendation. Anticoagulation with prophylactic dose of heparin is recommended for hospitalized COVID-19 patients. Collectively, anti-inflammation drugs, especially targeting IL-6, is the main therapeutic option in addition to antiviral agents.
From mechanistic points of view, it remains unclear whether dysregulated immune response is the primary pathogenic mechanism in severe COVID-19, although in the acute phase of severely ill patients with COVID-19, anti-inflammation agents are effective. In a retrospective observational study, persistent endotheliopathy with increased levels of factor VIII, VWF, and thrombomodulin, compared with healthy subjects, was observed in convalescent COVID-19 patients, independent of ongoing acute phase response or NETosis [215,216]. The hypercoagulable state observed in severe COVID-19 patients was associated with elevated levels of factor VIII and VWF, suggesting the involvement of endothelial cells [110]. In addition, the elevated levels of VWF were also observed in mild COVID-19 patients [95]. Together with milder thrombocytopenia in severe COVID-19 compared with severe SARS and MERS, these findings suggest that endothelial dysfunction might be the main contributor to the pathogenesis of COVID-19. On the other hand, systemic endothelial dysfunction without detectable viral RNAs in the bloodstream or in the endothelium suggests important roles of thromboinflammation in the pathogenic mechanisms of COVID-19 [217]. The vicious cycle of interaction between thromboinflammation and endothelial injury might contribute to disease severity. Contribution of each pathogenic mechanism to COVID-19 might differ among patients, depending on their background characteristics and the stages of disease progression. In fact, pre-existing cardiovascular risk factors increase the incidence of thromboembolic events in COVID-19 [35,36,37,38].
Activation of the kallikrein–kinin system due to endothelial dysfunction has been implicated in the pathophysiology of COVID-19 [125]. ACE2 internalization and/or factor XII activation might be involved in this process. However, it remains unclear which of the two mechanisms mainly contribute to activation of the kallikrein–kinin system in COVID-19. It was reported that systemic factor XIIa levels were not elevated in patients with long COVID-19 syndrome, compared with healthy subjects, while factor VIII and VWF levels were higher in these patients, suggesting that ACE2 internalization might be the main contributor to activation of the kallikrein–kinin system in COVID-19 [215]. ACE2 decoys and bradykinin inhibitors could be therapeutic candidates for COVID-19 that target endothelial dysfunction, in addition to statin and nitric oxide.
In summary, therapeutic strategies targeting thromboinflammation and endothelial injury in COVID-19 have been in development by repurposing currently available drugs, in addition to vaccines or antiviral drugs [218]. Considering the multifactorial pathogenic nature of COVID-19, combinatorial treatment might be necessary to improve clinical outcomes, including mortality. Further studies that clarify the pathogenic basis of COVID-19 might provide new insights in determining the optimal timing and combinations of drug administration as well as creating novel therapeutic strategies.
根据临床试验结果,目前对接受机械呼吸或循环支持的 COVID-19 重症患者的治疗建议是使用地塞米松。如果这些患者在入住 ICU 后 24 小时内,地塞米松加托珠单抗(一种 IL-6 抑制剂)可能是另一种选择。对于需要通过高流量设备或无创通气供氧的住院 COVID-19 患者的治疗,推荐地塞米松或地塞米松加瑞德西韦,而如果呼吸窘迫和全身炎症迅速恶化,则可以添加托珠单抗或 JAK 抑制剂巴瑞替尼在这些患者中观察到。对于需要补充氧气的住院轻中度 COVID-19 患者,建议使用地塞米松和/或瑞德西韦。对于没有氧气支持的 COVID-19 住院患者或门诊患者,治疗建议的证据不足。对于住院的 COVID-19 患者,推荐使用预防性剂量的肝素进行抗凝。总的来说,抗炎药物,尤其是针对 IL-6 的药物,是除抗病毒药物之外的主要治疗选择。

细胞培养摇瓶500ml
从机制的角度来看,免疫反应失调是否是重症 COVID-19 的主要致病机制尚不清楚,尽管在 COVID-19 重症患者的急性期,抗炎药是有效的。在回顾性观察研究,持续性内皮盲具有增加的因子VIII,VWF,和血栓调节蛋白的水平,与健康受试者相比,在恢复期COVID-19的患者,正在进行独立急性期反应的或NETosis [观察215,216 ]。在重症 COVID-19 患者中观察到的高凝状态与因子 VIII 和 VWF 水平升高有关,表明内皮细胞的参与 [ 110]]。此外,在轻度 COVID-19 患者中也观察到 VWF 水平升高 [ 95 ]。与严重的 SARS 和 MERS 相比,严重的 COVID-19 患者血小板减少症较轻,这些发现表明内皮功能障碍可能是 COVID-19 发病机制的主要因素。另一方面,在血流或内皮中没有检测到病毒 RNA 的全身性内皮功能障碍表明血栓炎症在 COVID-19 的致病机制中起着重要作用 [ 217]]。血栓炎症和内皮损伤之间相互作用的恶性循环可能会导致疾病的严重程度。根据患者的背景特征和疾病进展阶段,每种致病机制对 COVID-19 的贡献可能因患者而异。事实上,预先存在的心血管疾病的危险因素增加的血栓栓塞事件在COVID-19 [发病35,36,37,38 ]。
由于内皮功能障碍导致激肽释放酶-激肽系统的激活与 COVID-19 的病理生理学有关 [ 125 ]。ACE2 内化和/或因子 XII 激活可能参与此过程。然而,目前尚不清楚这两种机制中的哪一种主要有助于激活 COVID-19 中激肽释放酶-激肽系统。据报道,与健康受试者相比,长期 COVID-19 综合征患者的全身性因子 XIIa 水平没有升高,而这些患者的因子 VIII 和 VWF 水平更高,表明 ACE2 内化可能是激活COVID-19 中的激肽释放酶-激肽系统 [ 215]]。除了他汀类药物和一氧化氮外,ACE2 诱饵和缓激肽抑制剂可能是针对内皮功能障碍的 COVID-19 的治疗候选药物。
总而言之,除了疫苗或抗病毒药物外,通过重新利用当前可用的药物,已经开发出针对 COVID-19 中血栓炎症和内皮损伤的治疗策略 [ 218 ]。考虑到 COVID-19 的多因素致病性质,可能需要联合治疗来改善临床结果,包括死亡率。阐明 COVID-19 致病基础的进一步研究可能为确定最佳给药时机和组合以及创造新的治疗策略提供新的见解。








关键词:COVID-19; endothelial injury; inflammation; platelet activation; SARS-CoV-2; therapeutics; thrombosis
COVID-19;内皮损伤;炎症; 血小板活化;SARS-CoV-2;疗法;血栓形成
上一篇: 血清瓶的灭菌要求是怎样的
下一篇: 细胞工厂培养细胞常见的污染——化学污染