关键词: PD-L1 inhibitor; immune checkpoint blockade; immunotherapy
Targeting the programmed cell death protein 1/programmed cell death 1 ligand 1 (PD-1/PD-L1) interaction has become an established strategy for cancer immunotherapy. Although hundreds of small-molecule, peptide, and peptidomimetic inhibitors have been proposed in recent years, only a limited number of drug candidates show good PD-1/PD-L1 blocking activity in cell-based assays. In this article, we compare representative molecules from different classes in terms of their PD-1/PD-L1 dissociation capacity measured by HTRF and in vitro bioactivity determined by the immune checkpoint blockade (ICB) co-culture assay. We point to recent discoveries that underscore important differences in the mechanisms of action of these molecules and also indicate one principal feature that needs to be considered, which is the eventual human PD-L1 specificity.
靶向程序性细胞死亡蛋白 1/程序性细胞死亡 1 配体 1 (PD-1/PD-L1) 相互作用已成为癌症免疫治疗的既定策略。尽管近年来提出了数百种小分子、肽和拟肽抑制剂,但只有少数候选药物在基于细胞的检测中显示出良好的 PD-1/PD-L1 阻断活性。在本文中,我们根据 HTRF 测量的 PD-1/PD-L1 解离能力和免疫检查点阻断 (ICB) 共培养测定确定的体外生物活性比较了来自不同类别的代表性分子。我们指出最近的发现强调了这些分子作用机制的重要差异,也表明了一个需要考虑的主要特征,即最终的人类 PD-L1 特异性。
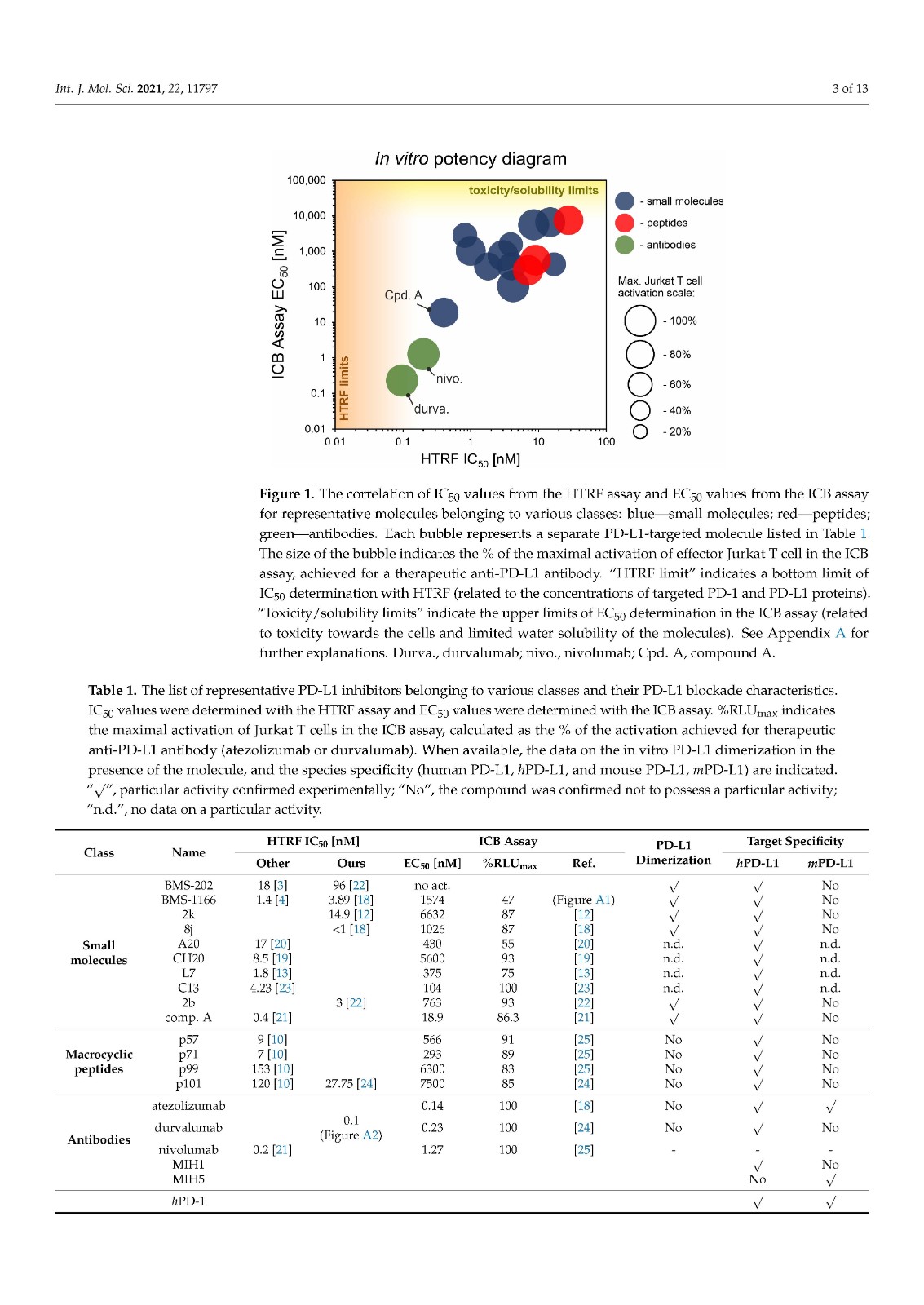
We performed a comparison of the in vitro targeting of the PD-1/PD-L1 immune checkpoint with molecules selected from various classes, including small molecules, macrocyclic peptides, and monoclonal antibodies. For this, two standardized and popular techniques used in the available literature were applied: the protein-based Homogeneous Time-Resolved Fluorescence (HTRF) method and the cell-based Immune Checkpoint Blockade (ICB) assay. Using these methods, three parameters describing the in vitro potency of the tested compounds were determined: (i) IC50 values of the blockade of PD-1/PD-L1 complex formation (from HTRF); (ii) EC50 values of the reactivation of the effector T cells blocked with PD-L1 (from ICB); and (iii) maximal activation levels of the effector T cells, calculated as the % of the activation in the presence of therapeutic anti-PD-L1 antibody atezolizumab or durvalumab. Based on these parameters, a bubble plot was prepared to visualize the differences between groups of molecules (Figure 1). The numeric data are also presented in Table 1.
我们对 PD-1/PD-L1 免疫检查点的体外靶向与选自各种类别的分子进行了比较,包括小分子、大环肽和单克隆抗体。为此,应用了现有文献中使用的两种标准化和流行技术:基于蛋白质的均相时间分辨荧光 (HTRF) 方法和基于细胞的免疫检查点阻断 (ICB) 检测。使用这些方法,确定了描述受试化合物体外效力的三个参数:(i) PD-1/PD-L1 复合物形成阻断的IC 50值(来自 HTRF);(ii) 欧共体50被PD-L1(来自ICB)阻断的效应T细胞的再激活值;(iii) 效应 T 细胞的最大活化水平,计算为在治疗性抗 PD-L1 抗体 atezolizumab 或 durvalumab 存在下的活化百分比。

血清培养基瓶500ml









来源:MDPI https://www.mdpi.com/1422-0067/22/21/11797/htm
上一篇: 怎样判断血清瓶中血清的质量
下一篇: 细胞工厂使用的具体操作步骤